Coronary Interventionism
Structural Cardiology
Embolization
Neuroradiology
Peripheral Interventionism
Biopsies
Tricvalve
Ultraseal Left Atrial Appendage Closure
Ultrasept Closure of Patent Foramen Ovale
SimValve Force
Lava
SIR-Spheres
Needles
Extension Cords
Syringes
Vascular Introducers
Hemostatic Keys
Hemostatic Patches
Inflation Devices
Radial Closure Devices
Diagnostic Guides
Guides
Guiding Catheters
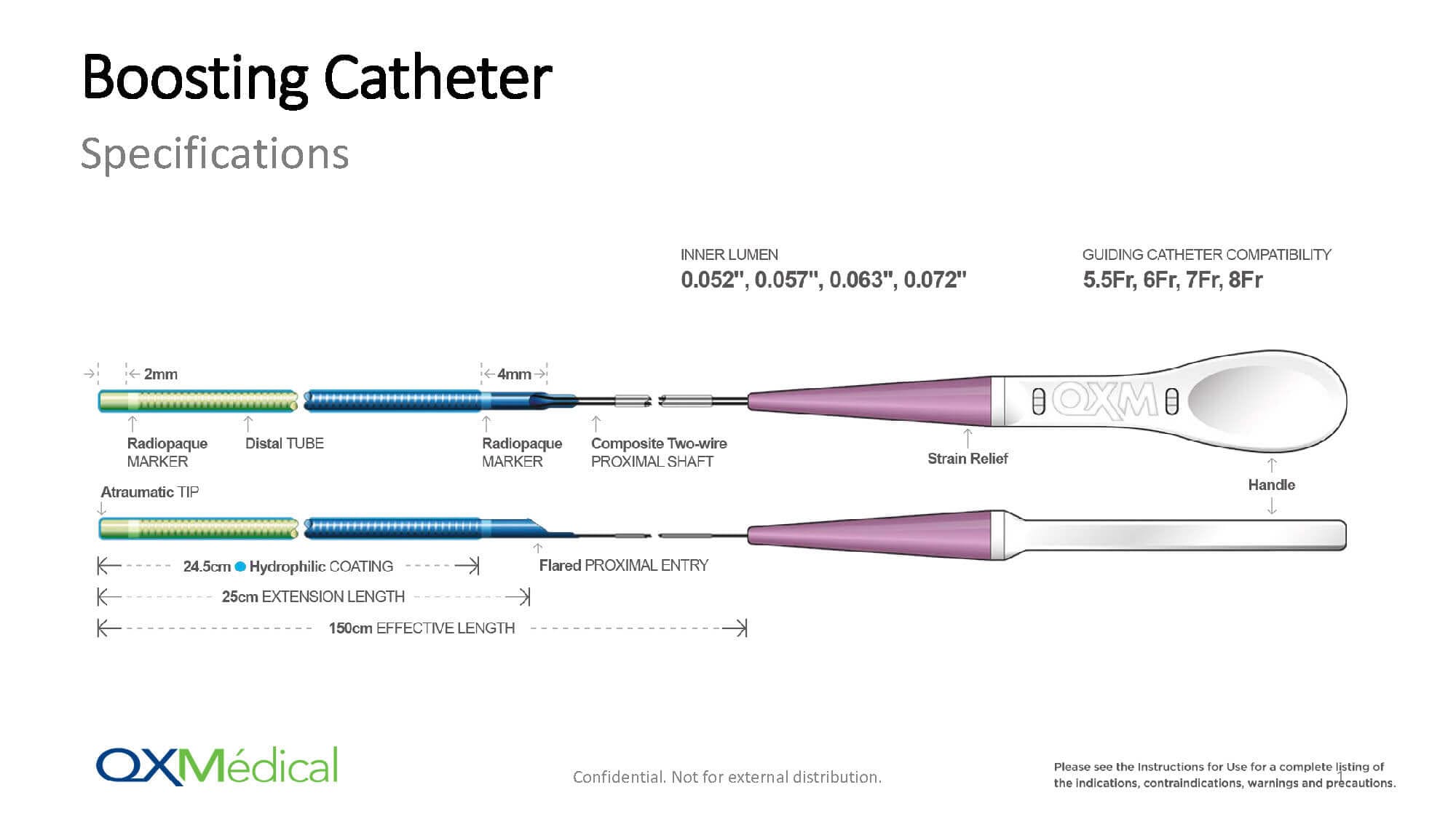
Distal Access
Microcatheters
Embolization
Remodeling
Remodeling Ball
Guides
Guiding Catheters
Distal Access
Microcatheters
Embolic Protection
DEB ball
Angioplasty Ballons
Guides
Guiding Catheters
Distal Access
Microcatheters
Thrombus Aspiration
Machanical Thrombectomy
Embolic Protection
Needles
Extension Cords
Syringes
Vascular Introducers
Hemostatic Keys
Hemostatic Patches
Inflation Devices
Radial Closure Devices
Diagnostic Guides
Endovascular Treatment
Angioplasty Balloons
Drug-active balloons
Interventionism Guides
Recanalization Microcatheter
Support Catheters
CTO catheter
Retrievable Stent System
Kronos